 |
Membrane Pharmacy Structure DynamicsResearch group : Priv.Doz. Dr. Thomas NawrothRegulation of ATP-syntase |
 |
Membrane Pharmacy Structure DynamicsResearch group : Priv.Doz. Dr. Thomas NawrothRegulation of ATP-syntase |
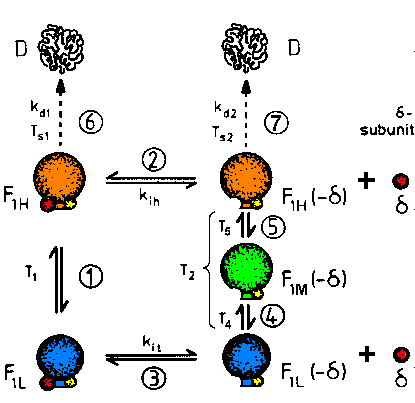 |
| Fig.1: The changes of structure and function of F1ATPase from Micrococcus luteus correspond to the dissociation of the inhibitory delta-subunit (reactions 2, 3), the reversible tempereature dependence (reactions 1, 4, 5) and to irreversible thermical denaturation (reactions 6, 7). Below the threshold temperatures Ts1 and Ts2 no significant denaturation is observed. This F1ATPase exists at lest in 5 enzymatically active modifications (conformations) (from T20, reference F8). |
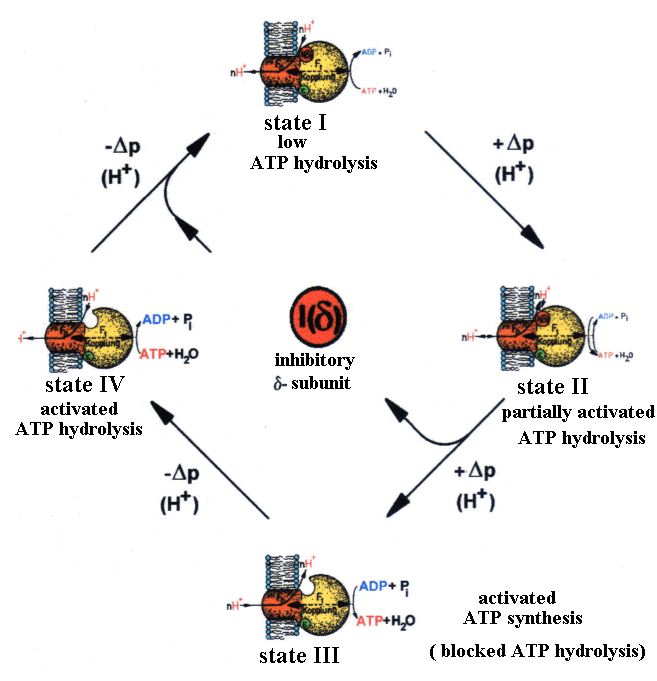 |
| Fig.2: The changes of structure and function of ATP-synthase from Micrococcus luteus corresponds at a given temperature and ATP/ADP-level to the membrane energization delta-p and the dissociation of the inhibitory delta-subunit (in E. coli : epsilon) (from T20). |